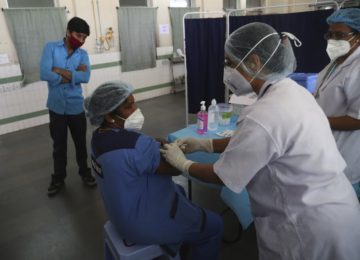
Vaccine rollout hits snag as health workers balk at shots
FILE – In this Jan. 7, 2021, file photo, a nurse puts on protective gear in a COVID-19 unit in California. The nation’s biggest immunization rollout in history is facing pushback from an unlikely source: health care workers who witnessed COVID-19′s devastation