Some European countries begin barring U.K. travelers
Widespread vaccinations at ravaged U.S. nursing homes are expected to begin this week. Apple is shuttering its California stores as the virus surges in the state. — NYT: Top Stories
Great f.y.i. Get hooked here!
Local, national & international business news and events.
Widespread vaccinations at ravaged U.S. nursing homes are expected to begin this week. Apple is shuttering its California stores as the virus surges in the state. — NYT: Top Stories
OLDWICK, N.J.–(BUSINESS WIRE)–AM Best has affirmed the Long-Term Issuer Credit Rating (Long-Term ICR) of “a-” and the Long-Term Issue Credit Ratings (Long-Term IR) of UnitedHealth Group Incorporated (UnitedHealth Group) (Minnetonka, MN) [NYSE: UNH]. AM Best also has affirmed the Short-Term Issue Credit
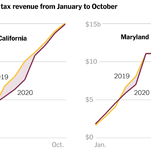
As the pandemic batters the poor, the rich prop up state budgets. — NYT: Top Stories
PARAMUS, N.J.–(BUSINESS WIRE)–Arena STEM, a new retail edutainment concept opened its first U.S. location at Westfield Garden State Plaza today. The flagship store will be located on level two near Nordstrom. Arena STEM is a new and innovative concept that provides an

City and state officials are trying to recover millions from quick deals made during the worst weeks of the pandemic this spring. — NYT: Top Stories
Application Based on Overall Survival and Progression-Free Survival Data Comparing KEYTRUDA Plus Chemotherapy to Chemotherapy Alone From Pivotal Phase 3 KEYNOTE-590 Trial KENILWORTH, N.J.–(BUSINESS WIRE)–$MRK #MRK–Merck (NYSE: MRK), known as MSD outside the United States and Canada, today announced that the U.S.
Environmental goals build on previously announced D&I commitments to enhance the company’s enterprise ESG efforts PRINCETON, N.J.–(BUSINESS WIRE)–Bristol Myers Squibb (NYSE: BMY) today announced it is strengthening its commitment to environmental sustainability on a global basis by setting new 2030 and 2040
Design Innovations Reduce Costs by Half, Decrease Production Time, and Increase Reliability of Spacecraft Solutions MONTVILLE, N.J.–(BUSINESS WIRE)–#JAXA—Marotta Controls, a rapidly growing aerospace and defense supplier based in New Jersey, today announced that it will be supplying critical system valves to Honeybee

Up to a foot of snow is forecasted in New York City between Wednesday afternoon and Thursday evening. — NYT: Top Stories